titanium
History
Titanium was discovered in Cornwall, England, in 1791 by William Gregor. It was also discovered around the same time by Hungarian mineralogist Franz-Joseph Müller von Reichenstein, and later in 1795 by German chemist Martin Heinrich Klaproth – who gave titanium its name, a reference to the Titans of Greek mythology.[1]
However, it was not until after 1932 that commercial use for titanium became possible, due to methods established by William Justin Kroll. Kroll devised ways of reducing titanium tetrachloride (TiCl4) into its metal form.[3] His process is still used today for commercially-produced titanium.[2]
The cost of titanium can be very high. This is ostensibly because the process of extracting titanium from its various ores is laborious and costly.[3] Although it is indeed expensive as an engineering material, it is far less expensive than the jeweler's usual precious metals, even silver. At the start of 2014, no prices for pure titanium or its common commercial alloys exceeded US $10 per pound. The process of machining titanium rings is expensive, and necessary since the metal is nearly impossible to craft by rolling or soldering in the way silver, gold, and even platinum are formed.[4]
The metal can be machined using the same equipment and via the same engineering processes as stainless steel.[5] The usual jewelry-making techniques of rolling and soldering are not practical for titanium, although they can be fabricated by welding in an inert atmosphere using, for example, a laser welder.
Titanium has become popular as a jewelry material due to its various unique properties. Titanium is biocompatible (often referred to as hypoallergenic), or non-toxic to the human body. Similarly, titanium rings will not react with wearers who suffer allergies to other jewelry materials.[6]
It is highly resistant to most causes of corrosion, including sea water, aqua regia, chlorine (in water), and some acids. It is soluble in concentrated acids, however.[7] Titanium rings are therefore practical jewelry for those who regularly swim in the ocean or chlorinated pools. This is in contrast to some traditional jewelry materials, such as silver, brass, and bronze, which are prone to tarnish or other deterioration.
Titanium has positively and diversely impacted mankind more than any single element. It has taken us to the depths of the ocean and to the far reaches of space. (85% of the space shuttle's structure is titanium.) It is placed inside our bodies and on our sporting equipment. It is only in the last few years that we have begun to explore the artistic benefits of this miraculous material.[8]
ORIGINAL FROM NATIONAL MUSEUM COLLECTION
RING
Designed by: Helena Edman
Material: Gold, titanium
Size 1,5 x 91,9 x 2,5 cm
Year: 1984
Photo credit: Greta Lindström / Nationalmuseum
This ring is made by Helena Edman and it made of alternating sheets of gold and titanium. The titanium has a green color since it has been anodized.
Anodization of titanium rings is the process whereby an oxide film is formed on the surface of the titanium via an electrolytic process to create color. In the case of titanium rings, this process is performed after it is machined into shape. Oxidation changes the ordinary titanium color (generally silver, depending on composition and processing) and increases corrosion-resistance. The anodization process is extremely simple to carry out: the piece is immersed in an electrolyte, cola is popularly used, and a DC voltage, around 100 V, is applied. The voltage controls the thickness, and thus the color, of the anodization.
Colors achievable through the anodization of titanium.
Dyes are not necessary to color anodized titanium. The color that results on a titanium ring depends on the thickness of the oxide coating, which is determined by the anodizing voltage. The image to the left shows the color spectrum range that can be achieved by anodizing. The colors, which are simply different wavelengths of light, arise from constructive interference between the light reflected from the surface of the oxide layer and light reflected from the metal surface below.
re-designed RING
RE-DESIGN
When I look at Edmans ring it make me think of a frozen movement, falling water. Edmans ring refers to tradition craft techniques with in jewelry tradition, in for of piercing, bending and riveting, which gives the piece a controlled and firm expression.
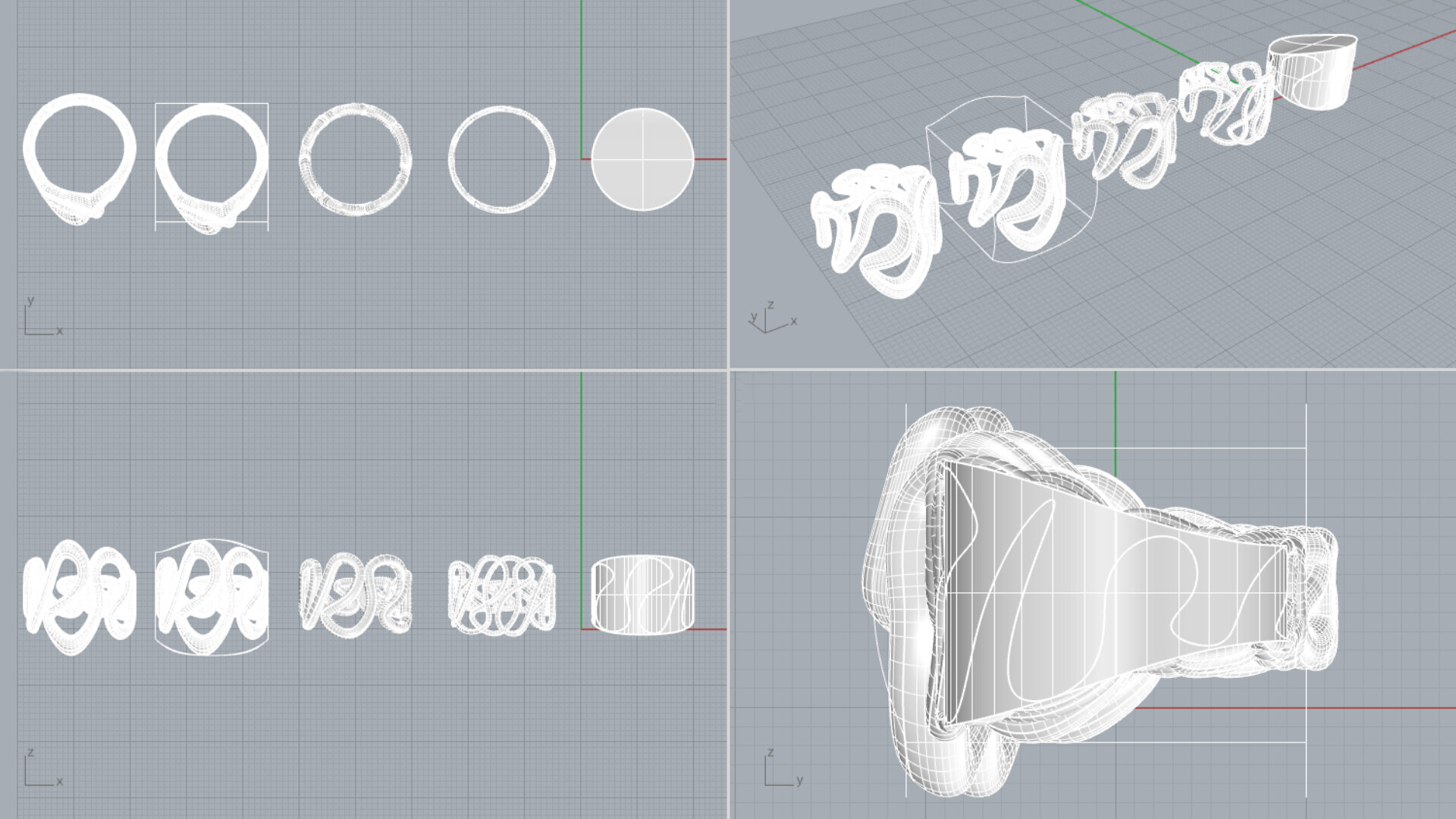
For my design I wanted to have a fluid expression, where the movement was captured by the hard titanium. Edman’s ring has a complex structure and is buildup of several different elements, I wanted to do the opposite and only use one element. I stared out by making a simple ring in CAD, I used the sketch tool to draw a continues poly line on the surface of the ring. It was hard to achieve a line that had gracefulness and freedom in a cad program. The lines that I made felt stiff and controlled and it took a lot of tryouts to succeed with these firs simple step.
Later the pipe command was used to give the poly line a thickness and finally, the cage edit tool was used to change the thickness of the ring’s different segments. The final ring has been 3d-printed in a titanium since that was the most efficient way to produce it.
process picture
3d-model of redesign
Footnotes
[1] Emsley, John (2001). "Titanium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 451–452. ISBN 0-19-850340-7.
[2] Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5
[3] Emsley, John (2001). "Titanium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 451–452. ISBN 0-19-850340-7.
[4] Barksdale, Jelks (1968). "Titanium". in Clifford A. Hampel (editor). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 734. LCCN 68-29938.
[5] Barksdale, Jelks (1968). "Titanium". in Clifford A. Hampel (editor). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 734. LCCN 68-29938.
[6] Emsley, John (2001). "Titanium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 451–452. ISBN 0-19-850340-7.
[7] Casillas, Norberto; Charlebois, Steven; Smyrl, William H.; White, Henry S. (1994). "Pitting Corrosion of Titanium". Journal of the Electrochemical Society. 141 (3): 636.
[8] https://www.titanium-jewelry.com/abouttitanium.htmloriginal from National museum collection
This project was made possible with the support of